Relationships among kinesiophobia, balance, disability, walking and functional abilities in Parkinson’s disease
Riassunto
L’obiettivo dello studio è: 1) valutare le relazioni tra cinesiofobia, equilibrio, disabilità, deambulazione e abilità funzionali nella malattia di Parkinson (PD); 2) valutare l’influenza della cinesiofobia, dell’equilibrio e della disabilità sul cammino e sulle abilità funzionali. 29 pazienti hanno completato la TSK, la BBS e la UPDRS-III. Inoltre, sono stati sottoposti ad un’analisi di cammino e mobilità funzionale. Sono state valutate le relazioni tra parametri clinico-strumentali. Le influenze di cinesiofobia, equilibrio e disabilità su velocità, lunghezza del passo e iTUG sono state valutate mediante regressione lineare multipla. La TSK è risultata correlata con HGS, per l’arto dominante e non dominante. La BBS ha mostrato correlazioni con TSK e iTUG. L’UPDRS-III è risultata correlata con BBS, TSK e iTUG. Velocità, lunghezza del passo e iTUG sono risultati significativamente influenzati dalla cinesiofobia, dall’equilibrio e dal grado di disabilità. I pazienti hanno mostrato alterazioni anche nella cinesiofobia, nell’equilibrio, nel cammino e nelle abilità funzionali.
Parole chiave: malattia di Parkinson, cinesiofobia, equilibrio, disabilità, cammino.
Abstract
The objective of the study is: 1) to evaluate the relationships between kinesiophobia, balance, disability, walking and functional abilities in Parkinson’s disease (PD); 2) to evaluate the influence of kinesiophobia, balance and disability on walking and functional abilities. 29 patients completed the TSK, BBS and UPDRS-III. In addition, they underwent an analysis of gait and functional mobility. The relationships between clinical-instrumental parameters were evaluated. The influences of kinesiophobia, balance and disability on gait velocity, stride length and iTUG were evaluated by multiple linear regression. TSK was found to be correlated with HGS, for the dominant and non-dominant limb. BBS showed correlations with TSK and iTUG. UPDRS-III was correlated with BBS, TSK and iTUG. Gait velocity, stride length and iTUG were significantly influenced by kinesiophobia, balance and disability. Patients also showed alterations in kinesiophobia, balance, gait and functional abilities.
Key words: Parkinson’s disease, kinesiophobia, balance, disability, walking.
Introduction
Parkinson’s disease (PD) constitutes a frequent neurodegenerative disease, with median annual incidence rates in high-income countries of 14/100000 people in the total population, with a rapid increase to 160/100000 in people older than 65 years1,2. The lifetime risk is estimated to be 2% for men and 1.3% for women1,3.
In older patients and those with several years since disease onset, motor disability is characterised by symptoms that include functional deficits, balance instability, walking disturbances, and difficulties in task-oriented abilities4. The formers include bradykinesia (i.e., slowness of movement), rigidity (i.e., stiff or inflexible muscles), and tremor (i.e., an involuntary quivering movement which typically starts in one hand, foot, or leg). Balance instability corresponds to the inability to balance due to loss of postural reflexes and it often leads to falls5,6. Walking deficits range from a reduced step and stride length to reduced cadence and velocity, and episodes of freezing of gait, resulting in altered mobility and falls7. Task-oriented abilities are frequently modified in patients with PD limiting usual daily living activities8,9. The aforementioned tasks include: functional movements of upper and lower limbs and the trunk; display actions such as reaching, grasping and manipulation, and sit-to-stand; and balance and walking as above described.
Interestingly, a maladaptive thought such as kinesiophobia may contribute to disability by enmeshing patients with PD in a downward spiral of increasing avoidance and disability10. According to the fear-avoidance model, negative appraisals (e.g., anxiety, catastrophizing or depression) due to a progressive disease induce fear-avoidance beliefs, which may lead patients to sacrifice everyday tasks and to use adaptive coping strategies (e.g., distraction, ignoring pain sensations, distancing from pain, or coping self-statements), definitely resulting in illness behaviour and poor physical performance11.
To the authors’ knowledge, these factors have never been investigated in a single study, despite multimodal rehabilitative programmes are increasingly advocated to address them in order to achieve better and long-lasting results in patients with PD12-17. Hence, the aim of this study was twofold: 1) to evaluate the relationships among kinesiophobia, balance, disability, walking and functional abilities in patients with PD, testing the hypothesis that they exhibit alterations in combination with a reduced propensity to move; and 2) to assess the influence of kinesiophobia, balance and disability on walking and functional abilities, testing the hypothesis that the former may affect the latter ones.
Materials and methods
This cross-sectional study was conducted at the Movement Disorders Unit, ARNAS G. Brotzu Hospital (Cagliari, Italy), where a sample of individuals with PD was recruited to take part in the tests. The inclusion criteria of the study were: diagnosis of idiopathic PD for at least 1 year, carried out by a neurologist according to the criteria of the United Kingdom PD Society Brain Banking18; Hoehn and Yahr (H&Y) score less than or equal to 3; ON phase with well-controlled disease in the aspect of motor symptoms; and fluency (i.e., ability to read and write) in Italian. The exclusion criteria were: the presence of mental health/psychiatric deficits (Mini-Mental State Examination score of <24); the presence of any other neurological or orthopedic disorder able to severely affect balance and mobility; systemic illness (including rheumatologic diseases); recent myocardial infarction, pulmonary disease or cerebrovascular event; and refusal to participate. Individuals who needed aids to ambulate (e.g. canes, walking frames, and crutches) were also excluded due to the reduced reliability of the instrumental measures of mobility for the specific setup here employed19.
The experimental protocol was approved by the local ethics committee (Prot. PG/2014/19654). All participants signed an informed consent form to take part in the study, which was conducted according to the ethical principles set out in the Helsinki Declaration of 196420 and its later amendments. Their demographic, anthropometric and clinical data are reported in Table 1.
Data acquisition and processing
Both clinical and instrumental assessments were carried out in a single session and two phases. In the first phase, participants were evaluated by the Tampa Scale of Kinesiophobia (TSK)10, the Berg Balance Scale (BBS)21,22 and the Unified Parkinson’s Disease Rating Scale (UPDRS) part III (motor performance)23. In the second phase, they underwent an instrumented analysis of gait, functional mobility and muscular strength, carried out using a wearable inertial sensor and a digital dynamometer, as described more in detail later. Personal and anthropometric data, and medical history for each subject were also collected in this phase.
Clinical scales
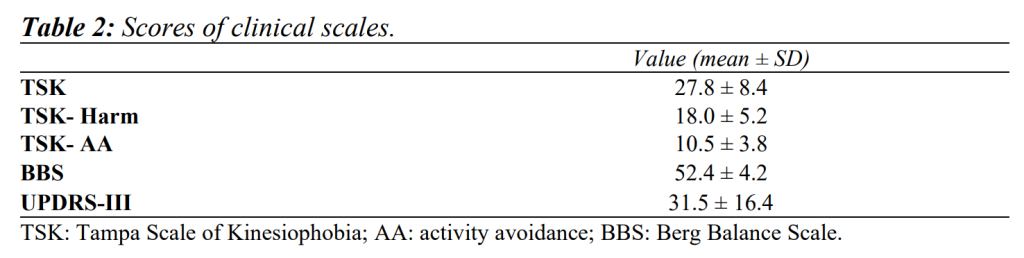
Kinesiophobia. It was assessed by the Italian 13-item version of the self-administered TSK with reversed items removed (items no. 4, 8, 12, 16); the process of cross-cultural adaptation has been previously described in detail and carried out in accordance with established guidelines10,24. The TSK consists of two subscales: harm (TSK-Harm, items no. 3, 5, 6, 7, 9, 11 and 13) and activity avoidance (TSK-AA, items no. 1, 2, 10, 14, 15, 17). Each item is scored using a four-point Likert scale ranging from 1 (strongly disagree) to 4 (strongly agree). The total and subscale scores have been calculated by adding the scores of individual items (ranges: 13–52; 7–28; and 6–24, respectively).
Balance. It was assessed by the Italian version of the BBS, which ranges from 0 (high risk of falling) to 56 (no risk of falling)25. The BBS has been identified as one of the best discriminators between fallers and non-fallers in subjects with PD, with a cutoff score of 43.526.
Disability. It was assessed by the Italian version of the UPDRS-III23,27, composed of 33 questions based on 18 items; each question has five possible answers related to widely used clinical terms: 0=normal, 1=slight, 2=mild, 3=moderate and 4=severe. The total score is calculated by adding the scores of the individual items and ranges from 0 (normal motor ability) to 132 (severe motor impairment).
Instrumented tests
Instrumental Gait Analysis. Participants’ gait was characterised using a wearable inertial sensor (G-Sensor, BTS Bioengineering, Italy), previously employed in studies involving individuals with PD28-31, which was attached at the lower lumbar level (centered on the L4-L5 intervertebral disc) using a semi-elastic belt. Participants were instructed to walk for 10 meters following a straight trajectory at a self-selected speed and in the most natural manner possible, while the device collected accelerations at 100 Hz frequency along three orthogonal axes: anteroposterior (AP), mediolateral (ML), and vertical (V). Data were transmitted in real-time via Bluetooth to a PC and subsequently processed using dedicated software (BTS G-Studio, BTS Bioengineering, Italy) in order to calculate the following spatial-temporal parameters: walking speed, cadence, stride length, stance, swing and double support phase duration (calculated as a percentage of the gait cycle). The software automatically removed the first and last two strides from the computation to consider only steady conditions, in order to remove the transient effect of acceleration and deceleration.
Instrumented Timed-Up-and-Go (iTUG) test. The iTUG test was performed using the same device, as previously described, but fixed at L1 vertebra location. To perform the test, participants were requested to sit on a standard office chair (seat height and width 48 cm, seat depth 40 cm) with seatback (34 cm high) and without armrests. Their arms were crossed at the wrists and held against the chest. Following a verbal start signal, they stood up from the chair, walked for 3 meters at a comfortable and safe velocity32 performed a 180° turn, around a sign marked on the floor, walked back to the chair and performed a second 180° turn to sit down. After an initial familiarisation trial, the second attempt was used for the analysis. As TUG is characterised by high test–retest reproducibility, a single trial can be considered sufficient to provide reliable data for both clinical and research purposes33. Even in this case, the three acceleration values (i.e., AP, ML and V) were acquired by the device at a 100 Hz and processed by the G-Studio software to obtain:
- iTUG: the overall time needed to perform the test (s);
- sit-to-stand time: the time necessary to pass from sitting to standing position (s);
- walk 1: the time needed to reach the mark placed at a 3m distance from the starting point (s);
- turn 1: the time required to perform the 180° turn necessary to reverse the gait direction (s);
- walk 2: the time needed to return from the mark to the starting point (s);
- turn 2: the time required to perform the 180° turn necessary to begin the sitting maneuver (s);
- stand-to-sit: the time needed to pass from standing to sitting position at the end of the task (s).
Handgrip strength test (HGS). It was measured using a validated34 digital hand dynamometer (DynEx, MD Systems, Westerville OH, USA). The participants were seated comfortably on a chair without armrests with their forearm leaning on a table in a neutral position, shoulder adducted and neutrally rotated, elbow fixed at 90°, and wrist between 0° and 30° of extension35. From this position, they were asked to squeeze the dynamometer with as much force as possible, while receiving verbal encouragement36. Three trials on each side, interspersed by approximately 20 seconds of rest and alternating sides were usually performed. The test was repeated if the difference in score among the three trials was found to be > 3 kgf. The final score was represented by the maximal grip value calculated from all six valid trials.
Statistical analysis
In order to assess the relationships between clinical and instrumented tests, the required sample size was calculated with the G*Power 3.1 (ρ of 0.5, power of 80% and type I error of 5%) and 29 patients were needed. Possible relationships between clinical and instrumented tests were assessed using the Spearman’s rank correlation coefficient by setting the significance level at p<.05. The correlation coefficient ρ was interpreted as follows: ρ<0.3 = low correlation; 0.3<ρ<0.7 = moderate correlation; ρ>0.7 = high correlation37.
We performed a multiple linear regression analysis through a blockwise modality to explore the influence of kinesiophobia, balance and disability with walking and functional abilities. Velocity and stride length are particularly indicative of walking38,39 and functional abilities40 in PD patients, so they were chosen as representative of walking performance, while iTUG was considered as functional ability. These three parameters were chosen, one at a time, as outcome measures of the multiple linear models. In the first block analysis, UPDRS-III, BBS and TSK-AA were entered in the model as independent variables; in the second block, an additional set of possible confounders (sex, comorbidities, marital status, and education level) was added as predictor (independent variables). An association was considered statistically significant if the p-value of the regression coefficient was <0.05. Once added individually in the regression model as an independent variable, potential confounders were considered relevant when they increased the regression coefficient of the main determinant of at least 10%.
The statistical analysis was performed using the software SPSS v23.0 (IBM, Armonk, NY).
Results
Of the 44 patients invited to participate, 7 did not meet the inclusion criteria (cognitive impairment, n=2; orthopedic disorders that severely affected balance and mobility, n=2, systemic illness, n=2; recent myocardial infarction n=1), 3 refused, and 5 were unable to adhere to the treatment (logistic problems, n=2; economic difficulties, n=1; personal problems, n=2).
The final sample consisted of 29 patients. Table 1 shows their clinical and socio-demographic characteristics.
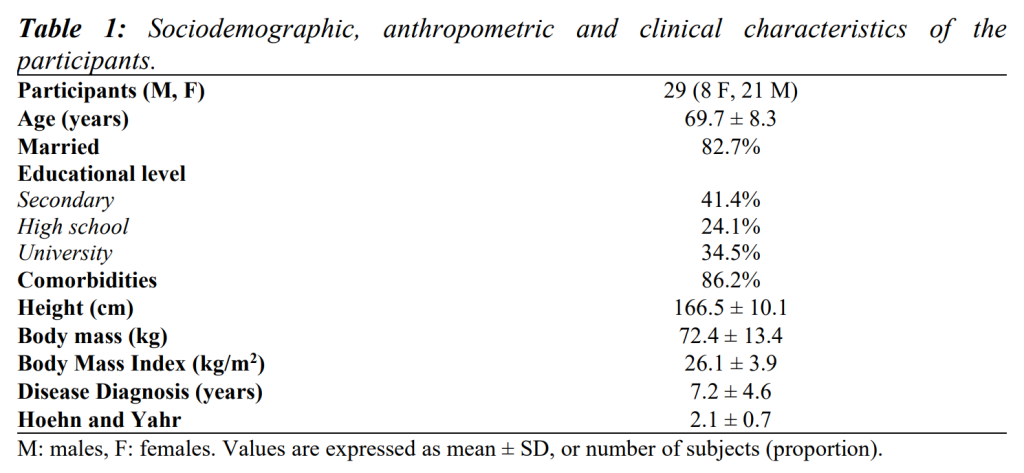
Table 2 summarises findings from clinical scales, and Table 3 shows results from gait analysis, iTUG and HGS.
UPDRS-III was negatively correlated with BBS (ρ=-.537, p<.01) and positively correlated with H&Y (ρ=.715, p<.01), TSK (ρ=.432, p<.05) and TSK-Harm (ρ=.419, p<.05). BBS was negatively correlated with H&Y (ρ=-.414, p<.05) and TSK (ρ=-.452, p<.05). TSK showed a positive correlation with H&Y (ρ=.375, p<.05).
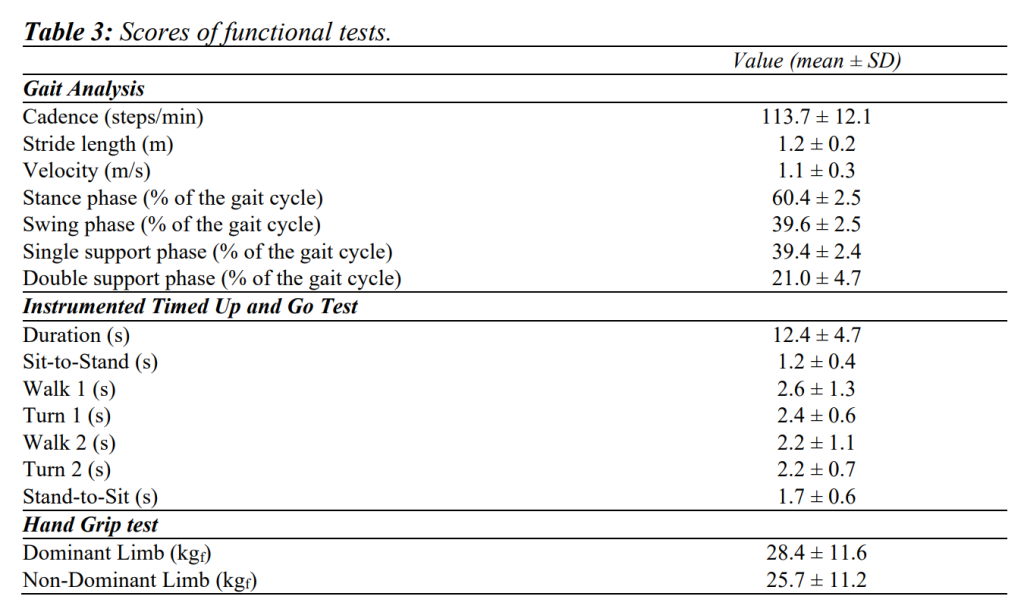
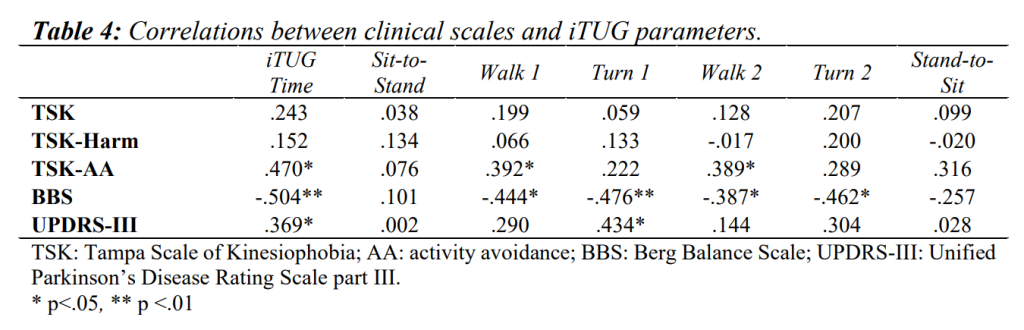
Table 4 and Table 5 summarise the correlations of clinical scales vs. iTUG and walking tests.
TSK-AA showed positive correlations with iTUG (ρ=.470, p<.05), walking 1 and 2 phases (ρ=.392, p<.05, ρ=.389, p<.05 respectively), and negative correlations with stride length (ρ=-.512, p<.01) and velocity (ρ=-.434, p<.05). TSK showed a negative correlation with HGS, for both dominant and non-dominant limbs (ρ=-.518, p<.01 and ρ=-.545, p<.01 respectively). TSK-Harm was mainly positively correlated with HGS for both dominant and non-dominant limbs (ρ=-.401, p<.05 and ρ=-.402, p<.05 respectively).
As for the relationships between BBS and iTUG tests, negative correlations were detected with overall iTUG (ρ=-.504, p<.01), walk 1, turn 1, walk 2 and turn 2 phases (ρ=-.444, p<.05; ρ=-.476, p<.01; ρ=-.387, p<.05 and ρ=-.462, p<.05 respectively). As for gait analysis, BBS showed positive correlations with cadence (ρ=.391, p<.05), stride length (ρ=.413, p<.05), velocity (ρ=.506, p< .01), single support phase (ρ=.476, p<.01) and swing phase durations (ρ=.445, p<.05), while negative correlations were found with the stance (ρ=-.445, p<.05) and double support phases durations (ρ=-.451, p<.05).
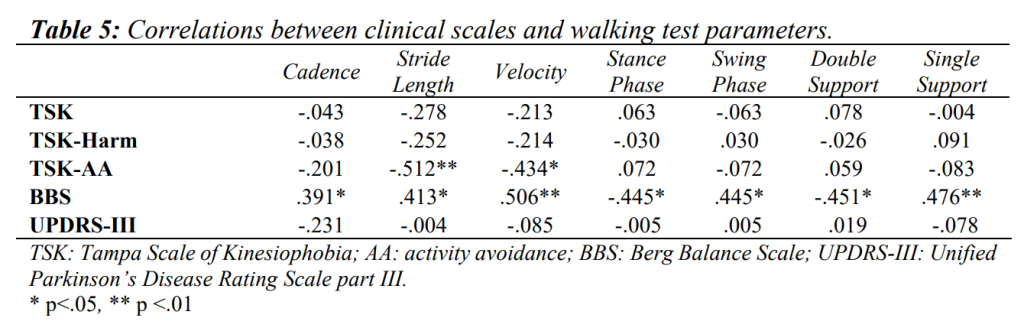
A positive correlation between UPDRS-III, and iTUG and turn 1 phase duration was found (ρ=.369, p<.05; ρ=.434, p<.05, respectively). Moreover, UPDRS-III item 3.9 (ability to stand up from a chair) was positively correlated with turn 1 and 2 phases (ρ=.470, p<.05; ρ=.452, p<.05), while 3.12 item (postural stability) was positively correlated with overall iTUG (ρ=.555, p<.01), walk 1 (ρ=.508, p<.01), and turn 1 and 2 phases (ρ=.548, p<.01; ρ=.484, p<.01). No significant relationships were detected between UPDRS-III and any of the walking parameters. Negative correlations were found between 3.9 and 3.12 UPDRS-III items and stride length and velocity (ρ=-.444, p<.05 and ρ=-.391, p<.05; ρ=-.416, p<.05 and ρ=-.455, p<.05 respectively for the former and the latter).
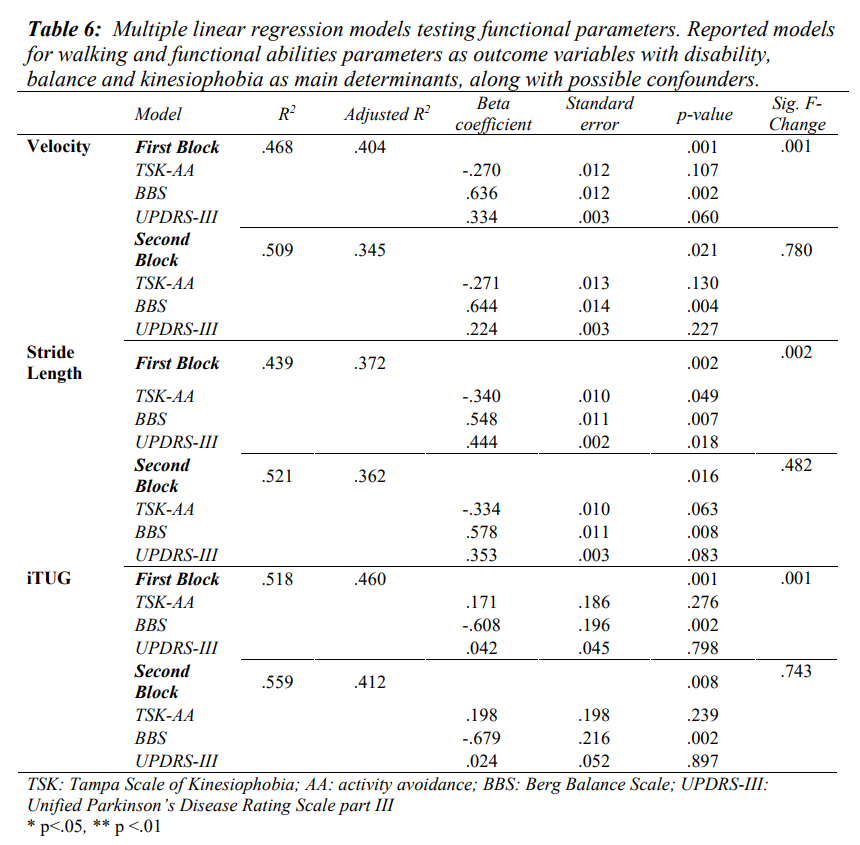
Multiple linear regression models showed kinesiophobia, balance and disability accounting for 47% of explained variance for walking velocity (r2=0.468; p<.001), 44% for stride length parameter (r2=0.439; p=0.002) and 52% for iTUG (r2=0.518; p<.001) (Table 6). When adjusting for possible confounders, the explained variance did not increase significantly (Table 6).
Discussion
The results of the present study substantially confirmed our hypotheses and could be analysed under two distinct aspects, namely the relationships existing between kinesiophobia, balance, disability, walking and functional abilities, and the influence of clinical scales on walking and functional abilities.
The correlation found between TSK and UPDRS-III suggests kinesiophobia is associated with the level of disability. Hence, kinesiophobia may contribute to persistent functional limitations, which in turn may enhance maladaptive thoughts when performing activities of daily living. A previous study investigated the relationships between TSK and UPDRS-III, and similar estimates were achieved10. TSK was also correlated with HGS: as patients with kinesiophobia present a reduction in handgrip strength, it can be argued fear-avoiders maladaptively reduce this motor ability to prevent the worsening of the disease. Despite no studies previously investigated this relationship in the same population, another study conducted in patients with chronic low back pain showed behavioural performance was significantly correlated with kinesiophobia41. TSK-AA was correlated with overall iTUG, iTUG walking phases, walking speed and stride length. Taken together, these findings suggest individuals with PD tend to adapt their gait patterns to compensate for alterations in sensory or motor systems associated with the disease. Such a strategy allows patients to achieve more stable locomotion and, probably, reduce the risk of falls. No previous studies investigated these relationships so comparisons cannot be made.
Participants exhibited reduced walking and functional performance when compared with age-matched unaffected peers reported in literature42-44. Indeed, they required a longer time to complete the iTUG test, and their gait was characterised by reduced velocity and stride length, and higher cadence, consistently with what observed in previous similar studies45,46. Moreover, results from instrumental tests were moderately to good correlated to BBS, thus confirming they encompass important information related to balance impairments associated with PD. These results are in line with those of previous studies, even from a quantitative point of view47-50. In particular, expanding the analysis to sub-phases time, it is noteworthy that BBS was associated with those tasks (walking and turning) more influenced by balance performance, while no significant correlations were detected as regards to sit-to-stand or stand-to-sit.
UPDRS-III was moderately correlated with iTUG, and two of its sub-items were correlated also with turn phases, consistently with previous studies51 which explain such association with the presence in UPDRS-III of specific items (i.e. rising from a chair and gait) which characterise the iTUG test. In contrast, no association between UPDRS-III and any of the walking parameters resulted from the analysis. Literature shows mixed findings as some studies reported a relationship between gait and either overall UPDRS score, UPDRS-III score or UPDRS-III derived scores, but others did not. Vieregge et al.52, Brusse et al.51 and Charness53 failed in detecting significant correlations with UPDRS (overall or part III), walking speed, cadence and stride length, probably because only a few UPDRS items refer to walking performance and bradykinesia during gait, and thus such scale is simply not adequate to fully represent the range of gait alterations typical of PD. However, few studies54,55 reported respectively an association between stride length and innovative sub-scores derived from UPDRS-III items, and between walking speed and UPDRS-III. It is possible differences with our findings may rely on the particular derived sub-scores obtained by Salarian et al.54, built starting from the UPDRS-III items, or on the particular population investigated in the case of Song et al.’s55 study. Indeed, the sample was composed only of individuals with a diagnosis of PD no older than 3 years55. At such an early stage of the disease, it is likely they did not show any particular alterations during the execution of motor tasks; in this regard, the same authors explained that timed functional performance measures in their sample were similar to those reported for an age-matched reference healthy population. On the other hand, we found correlations between 3.9 and 3.12 items of UPDRS-III with walking velocity and stride length. This suggests such specific items may refer to specific movement abilities representative of functional test performances.
As for the influence exhibited by kinesiophobia, balance and disability on walking and functional abilities, also kinesiophobia and balance should be included within the full evaluation of PD patients. It is worth of interest the association retrieved with stride length, whose performance should be considered when addressing general motor abilities of walking.
The above discussed results should be taken into account when evaluating patients with PD to highlight possible changes in functional tasks such as HGS, balance and gait, and in dysfunctional thoughts, such as kinesiophobia. Multidisciplinary cognitive-behavioural approaches should, therefore, include all these factors and rehabilitative interventions should be planned accordingly. Indeed, the use of exercises together with the management of dysfunctional thoughts and related behaviours is expected to contribute to boost the recovery, or at least to contribute to the maintenance of motor abilities in patients with PD and a subsequent improvement in disability.
Some limitations of the present study should be acknowledged. First, its cross-sectional nature implies the significant correlations here found should not be confused with causal effects. Second, since it was restricted to idiopathic PD patients, it is uncertain whether its findings can be extended to other neurodegenerative complaints, particularly parkinsonism disorders. Third, the clinical scales were tested in Italian patients, and it is uncertain whether the conclusions can be extended to different countries and cultures. Lastly, since gait and iTUG data acquired using inertial sensors might be less reliable when walking aids are employed, we were forced to exclude a relevant group of individuals with PD, who are also likely to be characterised by higher disability levels, and therefore results should be generalised with caution.
Conclusions
Patients who exhibited disability due to PD showed some alterations also in kinesiophobia, balance, walking and functional abilities. Kinesiophobia, balance and disability influence walking and functional abilities. These aspects should be taken into account when multidisciplinary rehabilitative interventions are planned.
References
- Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257-72.
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326-37.
- Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55(1):25-31.
- Kim HJ, Mason S, Foltynie T, Winder-Rhodes S, Barker RA, Williams-Gray CH. Motor complications in Parkinson’s disease: 13-year follow-up of the CamPaIGN cohort. Mov Disord. 2020;35(1):185-90.
- Palakurthi B, Burugupally SP. Postural instability in Parkinson’s disease: a review. Brain Sci. 2019;9(9):239.
- Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71(4):499-504.
- Nonnekes J, Nieuwboer A. Towards personalized rehabilitation for gait impairments in Parkinson’s disease. J Parkinsons Dis. 2018;8(s1):S101-6.
- McIsaac TL, Fritz NE, Quinn L, Muratori LM. Cognitive-motor interference in neurodegenerative disease: A narrative review and implications for clinical management. Front Psychol. 2018;9:2061.
- Silva PFS, Quintino LF, Franco J, Faria CDC de M. Propriedades de medida e de aplicabilidade de testes clínicos para avaliação do levantar/sentar em cadeira em indivíduos com doença neurológica: Revisão sistemática da literatura. Braz J Phys Ther. 2014;18(2):99-110.
- Monticone M, Ferrante S, Ambrosini E, Rocca B, Secci C, Foti C. Development of the Tampa Scale of Kinesiophobia for Parkinson’s disease: Confirmatory factor analysis, reliability, validity and sensitivity to change. Int J Rehabil Res. 2015;38(2):113-20.
- Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85(3):317-32.
- Post B, van der Eijk M, Munneke M, Bloem BR. Multidisciplinary care for Parkinson’s disease: not if, but how! Postgrad Med J. 2011;87(1031):575-8.
- van der Marck MA, Kalf JG, Sturkenboom IHWM, Nijkrake MJ, Munneke M, Bloem BR. Multidisciplinary care for patients with Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S219-23.
- Guo L, Jiang Y, Yatsuya H, Yoshida Y, Sakamoto J. Group education with personal rehabilitation for idiopathic Parkinson’s disease. Can. J. Neurol. Sci. 2009;36(1):51-9.
- White DK, Wagenaar RC, Ellis TD, Tickle-Degnen L. Changes in walking activity and endurance following rehabilitation for people with Parkinson disease. Arch Phys Med Rehabil. 2009;90(1):43-50.
- Tickle-Degnen L, Ellis T, Saint-Hilaire MH, Thomas CA, Wagenaar RC. Self-management rehabilitation and health-related quality of life in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2010;25(2):194-204.
- Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In-patient multidisciplinary rehabilitation for Parkinson’s disease: a randomized controlled trial. Mov Disord. 2015;30(8):1050-8.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-4.
- Zijlstra W. Assessment of spatio-temporal parameters during unconstrained walking. Eur J Appl Physiol. 2004;92(1-2):39-44.
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-4.
- Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the Berg Balance Scale for patients with Parkinson’s disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil. 2005;86(4):789-92.
- Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-Item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Phys Ther. 2008;88(6):733-46.
- Fahn S, Elton RL, UPDRS program members. Unified Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease, Vol 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. p 153-163.
- Monticone M, Giorgi I, Baiardi P, Barbieri M, Rocca B, Bonezzi C. Development of the Italian version of the tampa scale of Kinesiophobia (TSK-I): cross-cultural adaptation, factor analysis, reliability, and validity. Spine (Phila Pa 1976). 2010;35(12):1241-6.
- Ottonello M, Ferriero G, Benevolo E, Sessarego P, Dughi D. Psychometric evaluation of the Italian version of the Berg Balance Scale in rehabilitation inpatients. Europa Medicophys. 2003;39(4):181-9.
- Landers MR, Backlund A, Davenport J, Fortune J, Schuerman S, Altenburger P. Postural instability in idiopathic Parkinson’s disease: discriminating fallers from nonfallers based on standardized clinical measures. J Neurol Phys Ther. 2008;32(2):56-61.
- Antonini A, Abbruzzese G, Ferini-Strambi L, Tilley B, Huang J, Stebbins GT, et al. Validation of the Italian version of the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale. Neurol Sci 2013;34(5):683-7.
- Kleiner A, Galli M, Gaglione M, Hildebrand D, Sale P, Albertini G, et al. The Parkinsonian gait spatiotemporal parameters quantified by a single inertial sensor before and after automated mechanical peripheral stimulation treatment. Parkinsons Dis. 2015;2015:390512.
- Fastame MC, Hitchcott PK, Corona F, Pilloni G, Porta M, Pau M, et al. Memory, subjective memory and motor functioning in non-demented elders with and without Parkinson’s disease. Eur J Psychol. 2019;15(2):404-20.
- Pau M, Corona F, Pili R, Casula C, Guicciardi M, Cossu G, et al. Quantitative assessment of gait parameters in people with Parkinson’s disease in laboratory and clinical setting: are the measures interchangeable? Neurol Int. 2018;10(2):69-73.
- Bugané F, Benedetti MG, Casadio G, Attala S, Biagi F, Manca M, et al. Estimation of spatial-temporal gait parameters in level walking based on a single accelerometer: validation on normal subjects by standard gait analysis. Comput Methods Programs Biomed. 2012;108(1):129-37.
- Podsiadlo D, Richardson S. The Timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-8.
- Rydwik E, Bergland A, Forsén L, Frändin K. Psychometric properties of Timed Up and Go in elderly people: a systematic review. Phys Occup Ther Geriatr. 2011;29(2):102-25.
- Shechtman O, Gestewitz L, Kimble C. Reliability and validity of the DynEx dynamometer. J Hand Ther. 2005;18(3):339-47.
- Fess EE. Grip strength. In: Casanova JS. Clinical assessment recommendations. 2nd ed. Chicago: American Society of Hand Therapists; 1992. p. 41-45.
- Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222-6.
- Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. 5th ed. Boston: Houghton Mifflin; 2003. 756 p.
- Sidoroff V, Raccagni C, Kaindlstorfer C, Eschlboeck S, Fanciulli A, Granata R, et al. Characterization of gait variability in multiple system atrophy and Parkinson’s disease. J Neurol. 2021;268(5):1770-9.
- Schlachetzki JCM, Barth J, Marxreiter F, Gossler J, Kohl Z, Reinfelder S, et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS One. 2017;12(10):e0183989.
- Nocera JR, Stegemöller EL, Malaty IA, Okun MS, Marsiske M, Hass CJ. Using the timed up & go test in a clinical setting to predict falling in Parkinson’s disease. Arch Phys Med Rehabil. 2013;94(7):1300-5.
- Pfingsten M, Leibing E, Harter W, Kröner-Herwig B, Hempel D, Kronshage U, et al. Fear-avoidance behavior and anticipation of pain in patients with chronic low back pain: a randomized controlled study. Pain Med. 2001;2(4):259-66.
- Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture. 2011;34(1):111-8.
- Porta M, Pilloni G, Corona F, Fastame MC, Hitchott PK, Penna MP, et al. Relationships between objectively assessed functional mobility and handgrip strength in healthy older adults. Eur Geriatr Med. 2018;9(2):201-9.
- Mangano GRA, Valle MS, Casabona A, Vagnini A, Cioni M. Age-related changes in mobility evaluated by the timed up and go test instrumented through a single sensor. Sensors (Basel). 2020;20(3):719.
- Corona F, Pau M, Guicciardi M, Murgia M, Pili R, Casula C. Quantitative assessment of gait in elderly people affected by Parkinson’s Disease. In: 2016 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2016 – Proceedings. 2016.
- Van Lummel RC, Walgaard S, Hobert MA, Maetzler W, Van Dieën JH, Galindo-Garre F, et al. Intra-rater, inter-rater and test-retest reliability of an instrumented Timed Up and Go (iTUG) test in patients with Parkinson’s disease. PLoS One. 2016;11(3):e0151881.
- Schlenstedt C, Brombacher S, Hartwigsen G, Weisser B, Möller B, Deuschl G. Comparing the fullerton advanced balance scale with the mini-BESTest and berg balance scale to assess postural control in patients with Parkinson disease. Arch Phys Med Rehabil. 2015;96(2):218-25.
- Kobayashi E, Himuro N, Takahashi M. Clinical utility of the 6-min walk test for patients with moderate Parkinson’s disease. Int J Rehabil Res. 2017;40(1):66-70.
- Rodrigues Pereira NM, Massè Araya MJP, Scheicher ME. Analysis of correlation between instruments for evaluation of postural balance in institutionalized elderly. MOJ Gerontol Geriatr. 2019;4(2):69-72.
- Bennie S, Bruner K, Dizon A, Fritz H, Goodman B, Peterson S. Measurements of balance: Comparsion of the timed “up and go” test and functional reach test with theberg balance scale. J Phys Ther Sci. 2003;15(2):93-7.
- Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with Parkinson disease. Phys Ther. 2005;85(2):134-41.
- Vieregge P, Stolze H, Klein C, Heberlein I. Gait quantitation in Parkinson’s disease – Locomotor disability and correlation to clinical rating scales. J Neural Transm (Vienna). 1997;104(2-3):237-48.
- Charness AL. Correlations of Balance and Gait Measures with the UPDRS and with each other [dissertation]. [Galveston]: University of Texas Medical Branch; 2013. 356 p.
- Salarian A, Russmann H, Vingerhoets FJG, Dehollain C, Blanc Y, Burkhard PR, et al. Gait assessment in Parkinson’s disease: toward an ambulatory system for long-term monitoring. IEEE Trans Biomed Eng. 2004;51(8):1434-43.
- Song J, Fisher BE, Petzinger G, Wu A, Gordon J, Salem GJ. The relationships between the Unified Parkinson’s Disease Rating Scale and lower extremity functional performance in persons with early-stage Parkinsons’ disease. Neurorehabil Neural Repair. 2009;23(7):657-61.
Conflict of interest
The authors declare that there are no conflicts of interest relevant to this work.
Funding Sources
No specific funding was received for this work.
Authors’ contributions
Salvatore Simone Vullo contributed to the acquisition and interpretation of data for the work; drafted the work and finally revised it critically for important intellectual content; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Marco Monticone made substantial contributions to the conception and design of the work; contributed to the acquisition, analysis, and interpretation of data for the work; drafted the work and finally revised it critically for important intellectual content; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Federico Arippa made substantial contributions to the analysis, and interpretation of data for the work; drafted the work and finally revised it critically for important intellectual content; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Giovanni Cossu contributed to the interpretation of data for the work; revised the work critically for important intellectual content; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Massimiliano Pau made substantial contributions to the conception and design of the work; contributed to the analysis, and interpretation of data for the work; drafted the work and finally revised it critically for important intellectual content; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All authors read and approved the final version of the manuscript.
Acknowledgments
The authors wish to thank the hospital staff, the residents of the School in Physical Medicine and Rehabilitation, the students of the School of Physiotherapy, and the patients who took part in the study.